The CBC lab’s research focusses on the human brain, investigating both its structure (anatomy) and function (activity). In both aspects the emphasis is on the connected networks (circuits) in the brain and the interactions between groups of neurons in these circuits. The human brain consists of about 80 billion neurons, each of them making, on average, 10 to 20 thousand connections to other neurons. No other organ is so densely and intricately connected as the brain. The complex circuits formed by these connections support the communication of activity between neurons and, ultimately, the computations the brain can perform. In the CBC lab we use state-of-the-art 3D imaging methods to measure the connectivity in brain circuits at different spatial scales. We then model the activity and computations these circuits might support and relate these to measurements of human brain activity. We have a strong methods development component and develop hardware and software technology needed to answer basic and applied questions about human brain circuits and computations.
The human macroscale connectome
The human macroscale (cortical) connectome is the connection matrix of large sub-areas of the cortical surface through large cortical association projections. It can be characterized by diffusion MRI and tractography modeling methods. Tractography invoves modeling (‘tracking’) long-range connections from local fiber direction information, obtained from diffusion MRI. We have worked on the effect that different tractography algorithms have on the resulting connectome (Bastiani et al, 2012). We have also validated and fune-tuned diffusion MRI techniques and tractography algorithms using with gold-standard histology techniques applied to post-mortem tissue (Roebroeck et al., 2008 ; Seehaus et al., 2013).

Cortical layering and microciruit connectivity
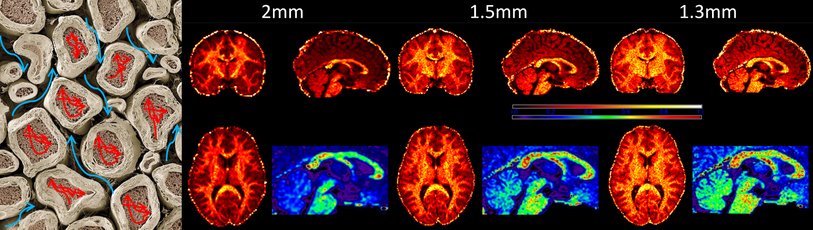
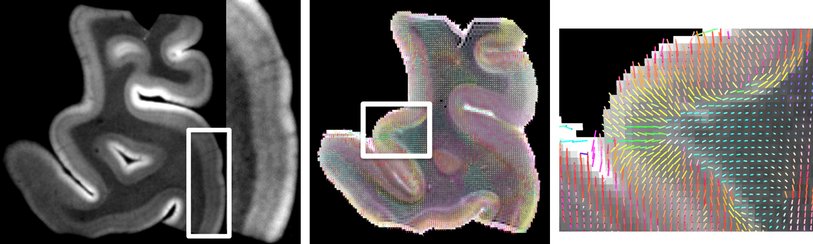
The cortical sheet that is connected over large distances by white matter pathways has its own structure at a finer scale. It contains many of the billions of neurons in a characteristic layered structure. Six separate layers can be distinguished in most areas over the 2-4mm thickness of the neocortical sheet, which contain different types of neurons in different proportions. Moreover, the radial (along the local depth direction of the curved cortex) and tangential (along the surface direction of the curved cortex) connections between these layered have characteristic structure. This connectivity forms local cannonical cortical microcircuits of a few tens of thousands of neurons in (less than) a square mm of cortical surface. We have worked on characterizing mesoscale (hundreds of microns) layered cortical structure with ex-vivo diffusion MRI and shown that we can delineate human cortical layers and the typical directions of neuronal connections in them (Roebroeck et al., OHBM 2012, Bastiani et al., ISMRM 2013). Further increasing the 3D resolution and coverage of diffusion MRI, and combining it with modern light microscopy techniques, promises an exiting new window on the cortical microcircuits that are thought to form the computational processing units of the human cortex.
Causal interactions of human brain regions
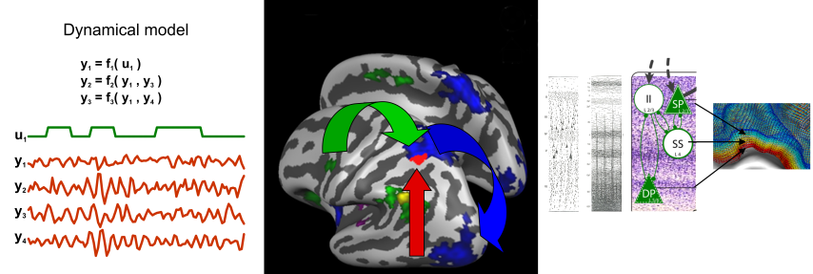
At the macroscale, large white matter projections support communication between specialized cortical areas of a few square centimeters. At a sufficiently large spatial and temporal scale, we can think of this communication to be ‘causal’, that is: one area influences or sends information to another. This large-scale abstraction surpasses mere correlation between area activity by assigning a direction to their communication. This can help identifying which cortical areas are interacting in a network for a certain task (e.g. stimulus-response association) and which are the dominant directions of infrmation flow between them. We have develop the technique of fMRI-based Granger causality mapping to do exactly this (Roebroeck et al., 2005) and reviewed the possibilities and challenges of causality models for fMRI (Roebroeck et al., 2009, 2011; Valdes-Sosa et al., 2011). With the advent of ultra-high field MRI (7T and above), the spatial resolution of fMRI is improving to the sub-millimeter level. This opens exciting new avenues of tapping into the activity of different parts of the cortical microcircuit (e.g. granular, infragranuar and supragranular) and use this to delineate information passing and computations performed at the finer intracortical scale (Roebroeck & Goebel, Cognitive Neurosciences V book, in press).
White matter microstructure
The large white matter projections between cortical areas in the macroscale cortical connectome are not just binary connections or even single scalar weighted connections. White matter axon bundles have many important microstructural parameters such as axon density, axon diameters and degree of myelination. All of these have a profound impact on the amount of information that can be transmitted and the speed with which signals can be propagated to other parts of the brain. Therefore measurement of these microstructural parameters is important to infer the functional and computational role of these projections. We work on applying biophysical compartment models to diffusion MRI data of the living human brain to get access to axonal density and, possibly, axonal diameter distributions.